Published online Apr 21, 2014. doi: 10.3748/wjg.v20.i15.4300
Revised: January 20, 2014
Accepted: February 17, 2014
Published online: April 21, 2014
With advances in the management and treatment of advanced liver disease, including the use of antiviral therapy, a simple, one stage description for advanced fibrotic liver disease has become inadequate. Although refining the diagnosis of cirrhosis to reflect disease heterogeneity is essential, current diagnostic tests have not kept pace with the progression of this new paradigm. Liver biopsy and hepatic venous pressure gradient measurement are the gold standards for the estimation of hepatic fibrosis and portal hypertension (PHT), respectively, and they have diagnostic and prognostic value. However, they are invasive and, as such, cannot be used repeatedly in clinical practice. The ideal noninvasive test should be safe, easy to perform, inexpensive, reproducible as well as to give numerical and accurate results in real time. It should be predictive of long term outcomes related with fibrosis and PHT to allow prognostic stratification. Recently, many types of noninvasive alternative tests have been developed and are under investigation. In particular, imaging and ultrasound based tests, such as transient elastography, have shown promising results. Although most of these noninvasive tests effectively identify severe fibrosis and PHT, the methods available for diagnosing moderate disease status are still insufficient, and further investigation is essential to predict outcomes and individualize therapy in this field.
Core tip: Chronic liver disease is a heterogeneous and dynamic condition. So, noninvasive exact estimations of the status and changes in hepatic fibrosis and portal hypertension are essential in the management of this disease. Recently, a few tests, such as liver stiffness measurement based on transient elastography (TE) or magnetic resonance have shown promising results in this field. However, the reproducibility of these non-invasive tests needs to be validated in diverse clinical situations and etiologies. Especially, it is important to study about the long term prognostic value of non-invasive tests in hepatic fibrosis and portal hypertension that can leads to new paradigm in the tailored management of chronic liver disease. Notably, the integration of serologic tests with other non-invasive tests, such as TE, allows the progression in the management of patients with chronic liver disease in the future.
- Citation: Kim MY, Jeong WK, Baik SK. Invasive and non-invasive diagnosis of cirrhosis and portal hypertension. World J Gastroenterol 2014; 20(15): 4300-4315
- URL: https://www.wjgnet.com/1007-9327/full/v20/i15/4300.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i15.4300
Regardless of its underlying etiology, fibrosis is the main component of chronic liver damage that directly relates to the severity and prognosis of the disease. Hepatic fibrosis and its secondary result, portal hypertension (PHT) are currently viewed as a dynamic process that can be reversible in some situation, if the underlying insult that has caused the fibrosis and cirrhosis has been removed[1]. Over time, the excess fibrous tissue of cirrhotic liver may also regress. Therefore, an accurate estimation of the severity of fibrosis and PHT is essential to evaluate the disease state and prognosis and is the first step towards the optimization of the treatment and estimation of its response so, the diagnosis of the heterogeneity of cirrhosis and PHT has become even more important for effective treatment[2,3].
Liver biopsy is the gold standard for assessing fibrosis. However, standard liver biopsy procedures have several limitations, including sampling errors, inter- and intraobserver variability, and invasiveness[4].
PHT, a frequently presenting clinical syndrome, is defined as a pathological increase in portal venous pressure between the portal vein and the inferior vena cava to higher than the normal range (≤ 5 mmHg). Increased portal pressure is the main factor determining the clinical course of cirrhosis. Presently, the favored method for determining portal venous pressure involves the catheterization of the hepatic vein and the measurement of the hepatic venous pressure gradient (HVPG). HVPG is one of the best surrogate markers in chronic liver disease; however, the measurement of the HVPG also has limitations, such as its invasiveness[5].
The ideal noninvasive test for diagnosing fibrosis and PHT should be simple and reproducible, readily available, less expensive than a biopsy, and able to predict the full spectrum of fibrosis and reflect any changes induced by therapy[6]. Moreover, recent advances in knowledge and treatment have led to proposals for more detailed histological diagnoses of fibrosis and these have made it even more difficult to find an ideal noninvasive substitute for liver biopsy[7,8]. In addition, it should be predictive of long-term outcomes related with fibrosis and PHT to allow prognostic stratification. Recently, many trials aimed at identifying such a test have been conducted, but no test has yet satisfied all of the aforementioned requirements.
In this report, we review the role of the gold-standard methods for the estimation of hepatic fibrosis and PHT and also recently studied alternative, non-invasive methods with suggestion of future directions.
Cirrhosis is defined by anatomical changes within the liver parenchyma, including fibrosis and the development of regenerating nodules, and its assessment is based on histologic examination. A liver biopsy is considered the gold standard for the diagnosis of cirrhosis. In general, histologic scoring systems assess the grade and stage of chronic hepatitis. The grade is used as a measure of necro-inflammatory activity, and the stage is used as a measure of fibrosis and architectural changes. These scoring systems use scales of four (1-4), five (0-4), or seven (0-6) stages for chronic hepatitis[7,9-12]. For biopsies, an adequate specimen needs to have a length of at least 1.5 cm or the presence of six to eight portal tracts[4]. However, the width of the biopsy core is also important because the hepatic architecture is difficult to appreciate in thin biopsies[13], and biopsy specimens obtained from subcapsular locations generally contain more fibrous tissue, which should be considered.
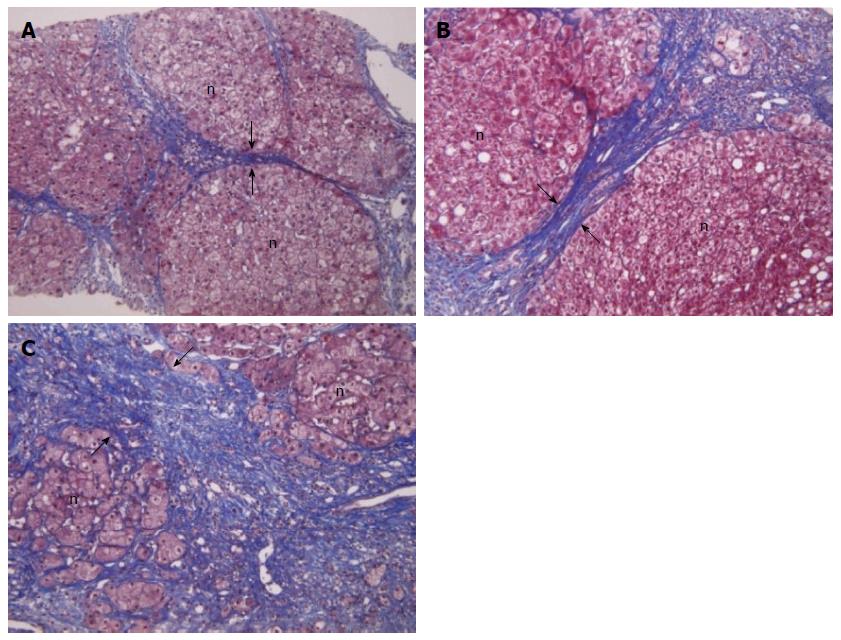
However, in clinical practice, liver biopsies have important limitations, such as invasiveness, potential for complications, sampling error, and inter- and intra-observer variability. Another important limitation is that a biopsy only represents approximately 1/50000 of the liver parenchyma[14]. Furthermore, the histologic diagnosis of cirrhosis maybe impossible in cases with large parenchymal nodules or so-called incomplete septal cirrhosis. However, despite these limitations, liver biopsy and histologic analysis have revolutionized the assessment of disease severity in chronic liver disease. Hepatic fibrosis is currently viewed as a dynamic process, in some cases, even the excess fibrous tissue of cirrhotic liver may regress over time. Distinguishing between the amount of hepatic fibrosis and the disease stage, which is related to both fibrosis and architectural changes, is important for the assessment of the effects of antifibrotic treatments. Nevertheless, a one-stage description of cirrhosis is inadequate, and subclassification has potential clinical utility[15,16]. From the aspect of histology, such subclassifications might allow better the prediction of prognosis and be helpful clinically. A few recent studies have shown the possibility of the clinical application of histological subclassification. In these studies, fibrosis scores, derived from nodule size and septal thickness, showed a significant correlation with the clinical stage[8] and prognosis[17] as well as the HVPG (Figure 1)[18,19].
Currently, the proportion of the liver biopsy specimen occupied by collagen (collagen proportion area, CPA) on Picro-Sirius stained histologic sections, as assessed by computer-assisted digital image analysis, has emerged as a useful continuous variable for the quantitative assessment of fibrosis in various clinical situations. The CPA measurement is based on the segmentation of digital images to measure the area occupied by collagen compared with the entire area of liver tissue[20,21]. The CPA has been reported to correlate with the HVPG in liver transplant recipients with hepatitis C virus infections, with or without cirrhosis, and the CPA measured at 1-year post-transplantation was found to be a predictor of clinical decompensation in later years[21]. This parameter has also been found to correlate with the liver stiffness (LS) measurements, as assessed by transient elastography (TE), in patients with chronic viral hepatitis[22,23]. Therefore, the quantitative assessment of hepatic fibrosis in liver biopsy specimens is promising as a prognostic marker and as a means to validate other noninvasive markers of fibrosis.
The measurement of the HVPG is the gold standard technique for the evaluation of PHT in liver disease. The HVPG is the difference between the wedged hepatic venous pressure (WHVP) and the free hepatic venous pressure (FHVP). The WHVP is measured by occluding the hepatic vein as stopping the blood flow causes a static column of blood to be formed, which is equal in pressure to the hepatic sinusoids. FHVP is a measure of the pressure of the non-occluded hepatic vein. In cirrhosis, WHVP provides an accurate estimate of portal pressure, as has been demonstrated in both alcoholic and viral cirrhosis patients[5,24].
In patients with cirrhosis, HVPG measurements provide independent prognostic information on survival and the risk of decompensation. Clinically significant PHT (CSPH, HVPG ≥ 10 mmHg) is necessary for the formation of esophageal varices, its bleeding, and the development of decompensation[15,25-27]. In patients who have already developed decompensation, HVPG provides predictive information about the risk of mortality in the future[28]. HVPG can also reflect liver parenchymal function[29], and correlates with the degree of histological liver fibrosis[8,24,30]. In addition, in some studies on HCV-related fibrosis and cirrhosis, HVPG showed a good correlation with the therapeutic response which suggested that the repeated HVPG measurements could be helpful in estimating the progression or regression of cirrhosis in patients with advanced hepatitis C virus (HCV)-related chronic liver disease[31,32]. HVPG also showed usefulness in identifying those patients who were at highest risk of decompensation by the recurrence of severe hepatitis C among those who had undergone post-liver transplantation for HCV-related cirrhosis[33-35]. CSPH is an independent predictor of the risk of developing hepatocellular carcinoma (HCC)[36], and it also increases the risk of liver failure and death after liver resection in patients with compensated chronic liver disease or hepatocellular carcinoma[37,38]. However, in case of non-cirrhotic portal fibrosis (NCPF) and extra-hepatic PV obstruction (EHPVO) which present only with features of PHT without any evidence of significant parenchymal dysfunction, HVPG is normal in EHPVO, whereas it is normal or slightly elevated in NCPF (median 7 mmHg). So, HVPG is not useful in the estimation of the severity of PHT and EV bleeding risk in EHPVO or NCPF[39].
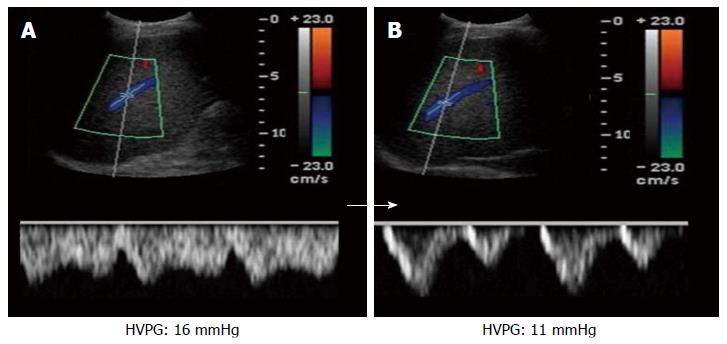
The HVPG response to pharmacological therapy enables the identification of those patients with PHT who are most likely to benefit from treatment. The development of esophageal varices is less likely if the HVPG is < 10 mmHg. The development of variceal bleeding and the presence of ascites have been known to occur when the HVPG is ≥ 12 mmHg[40]. If the HVPG falls to ≤ 12 mmHg with drug therapy, such as nonselective β blockers, the development of variceal bleeding can be prevented, and varices that are already present may decrease in size[41,42]. However, even if this target is not achieved, a decrease in the HVPG of ≥ 20% from baseline levels has shown similar effects to having an HVPG of < 12 mmHg[41]. In addition, this response markedly decreases the rebleeding risk in secondary prevention[42]. In addition, a good hemodynamic response was independently associated with a decreased risk of ascites and spontaneous bacterial peritonitis on follow-up and was an independent predictor of survival[43,44].
The evaluation of the acute HVPG response to intravenous propranolol therapy has been known to be useful for predicting the efficacy of nonselective β blockers at preventing the first bleed or rebleeding[45,46]. Furthermore, the acute HVPG response to propranolol is independently associated with survival in patients with cirrhosis and PHT[45].
However, although the HVPG measurement procedure is very well tolerated, its invasiveness and limited availability at hospitals has led to attempts to develop noninvasive alternatives, such as laboratory tests, imaging techniques, and LS measurements[47,48]. However, there has been no noninvasive alternative that can replace the HVPG measurement until now.
Because of the attractiveness, such as offering a sampling that reflects the whole liver, allowing repeated testing, reducing invasiveness, and increasing simplicity, many hematological and biochemical serum markers of fibrosis have been studied. In general, serum markers of fibrosis can be divided into two groups: “indirect markers”, which reflect the degree of fibrosis indirectly, and “direct markers”, which directly measure liver matrix components or enzymes that participate in matrix regulation[49].
Indirect markers in blood test include parameters related to cell lysis or inflammation (AST and ALT), cholestasis (γGT and bilirubin), hepatocyte synthetic function (INR, cholesterol, ApoA1, haptoglobin, and N-glycans), and hypersplenism due to PHT (platelet count)[49]. Many scores or indices of indirect parameters have been studied and the Fibrotest [α2-macroglobulin (α2M), ApoA1, bilirubin, γGT, and haptoglobin combination[50] and AST to platelet ratio index (APRI)][51] have been the most widely validated. In some studies, these indices have been shown to be good predictors of fibrosis and cirrhosis (AUROC = 0.92, 0.80, respectively)[52,53], but in a meta-analysis of chronic hepatitis C[54], they lacked reliability in the discrimination of the fibrosis stage.
Direct markers allow the quantitative assessment of the total amount of the hepatic extracellular matrix and its deposition or removal[51]. To improve accuracy, the use of a combination of markers is preferable to the use of a single marker[55], and various panels of direct markers have been described.
The original European Liver Fibrosis panel is an example of such a panel of biomarkers shown to be accurate in diagnosing significant fibrosis in a large, mixed liver disease population[56]. This panel incorporates hyaluronic acid, tissue inhibitor of matrix metalloproteinases-1, procollagen type III propeptide and age. The panel has since been simplified by removing age while maintaining diagnostic accuracy, as the enhanced liver fibrosis (ELF) test, which has been shown to be accurate in predicting significant liver fibrosis in independent populations[57-60]. ELF score predicts liver outcomes, with people having the highest ELF scores being significantly more likely to have clinical outcomes than those in lower-score groups with hazard ratios of 75 (ELF score 12.52-16.67), 20 (10.426-12.51) and 5 (8.34-10.425) compared with patients with ELF < 8.34[61]. In non-alcoholic fatty liver disease (NAFLD), one study reported favorable results of AUROC = 0.90, sensitivity 80%, and specificity 90% for advanced liver fibrosis, but this test needs to be further investigated[58].
A Cytokeratin-18 fragment in plasma indicates the apoptosis of liver cells and is the marker of NASH[62-64]. Plasma cytokeratin-18 fragment levels increased significantly in patients with steatohepatitis compare to normal or simple fatty liver patients. CK-18 fragment level showed a possibility as a screening test for NASH by showing favorable results (sensitivity 78%, specificity 87%, AUROC = 0.82) in meta-analysis[62]. However, this marker is currently unavailable in the clinical practice yet and the standard cut-off value for diagnosis also has to be established.
NAFLD Fibrosis Score (NFS) is one of the most widely-studied biochemical panels for the diagnosis of fibrosis in NAFLD, and it is composed of 6 markers (age, BMI, diabetes/impaired glucose tolerance, platelet count, albumin level, AST/ALT ratio) which are clinically or biochemically measured and calculated easily in the website (http://nafldscore.com). NFS has two cut-off values, [< -1.455 (low probability) and > +0.676 (high probability)] in evaluating liver fibrosis. According to a meta-analysis of 13 studies performed on 3064 patients, NFS showed a high AUROC value of 0.85 in diagnosing liver fibrosis greater than stage F3. By taking < -1.455 as cut-off, sensitivity was 90% and specificity was 60% in excluding advanced liver fibrosis. By taking > +0.676 as cut-off, sensitivity was 67% and specificity 97% in diagnosing advanced liver fibrosis. However, about 20%-58% of patients showed middle values between those two cut-off values (indeterminate probability), so in these case, liver biopsy has to be considered[62,65].
About the noninvasive estimation of PHT using laboratory test, just a few studies have been reported. Direct markers such as serum laminin levels, serum hyaluronic acid and procollagen type III propeptide were evaluated in an old small population studies and laminin and hyaluronic acid showed correlation with HVPG, however these markers has limitations in clinical application because of low predictive values for the presence of severe PHT and EVs[66-68]. In case of Fibrotest, it has a significant correlation with HVPG (Pearson correlation coefficient = 0.58), however, the correlation was weaker in cirrhosis patients (Pearson correlation coefficient = 0.24) and the AUROC for the diagnosis of severe PHT (HVPG > 12 mmHg) was only 0.79[69]. Additional study is needed to estimate the clinical usefulness of Fibrotest for the diagnosis of PHT. The platelet count, and the ratio of the platelet count to spleen diameter have shown to be able to exclude the existence of EVs, however, in the other studies, the results were not constant[70-72]. In case of APRI score also showed low AUROC in recent study in the prediction of the presence of EVs (EVs 0.62, large EVs 0.71)[73,74].
In summary, noninvasive laboratory markers are still insufficient for evaluating the dynamic changes in fibrosis and especially in PHT, and wider validations in various clinical situations are needed (Table 1).
| Biomarkers | Component | Etiologies | Patients (n) | F ≥ 2 (%) | F4 (%) | AUROC ≥ F2 | AUROC F4 |
| FibroTest[52] | GGT, haptoglobin, bilirubin, | HCV | 339 | 80 | - | 0.87 | - |
| apolipoprotein A1, alpha-2-macroglobulin | |||||||
| Forns Index[162] | Age, GGT, cholesterol, platelets | HCV | 476 | 26 | 0.81 | - | |
| APRI[53] | AST, platelets | HCV | 270 | 50 | 17 | 0.80 | 0.89 |
| ELF[56] | N-terminal propeptide of collagen type III, | Mixed | 1021/4961 | 40 | 12 | 0.78 | 0.89 |
| hyaluronic acid, TIMP-1, age | |||||||
| Hepascore[163] | Age, sex, alpha-2-macroglobulin, | HCV | 211 | 57 | 16 | 0.82 | 0.89 |
| hyaluronate, bilirubin, GGT | |||||||
| Fibrometer[164] | Platelets, prothrombin time, macroglobulin, | Mixed | 598/5032 | 56 | 37 | 0.89 | 0.81 |
| AST, hyaluronate, age, urea | |||||||
| FIB-4[165] | Age, ALT, AST, platelets | HCV | 847 | - | 17 | - | 0.85 |
| Fibroscan[110] | HCV | 183 | 74 | 25 | 0.83 | 0.95 | |
| Fibroscan[115] | HBV | 173 | 50 | 8 | 0.81 | 0.93 | |
| ARFI imaging[134] | Mixed | 146/871 | 48 | 16 | 0.89 | 0.979 | |
| MRE[161] | Mixed | 0.98 | 0.99 |
In chronic liver disease, the imaging diagnosis plays several significant roles in patient management, both in terms of diagnosing hepatocellular carcinoma and predicting its progression to cirrhosis. The basic diagnostic imaging modalities consist of ultrasound (US), computed tomography (CT), and magnetic resonance (MR) based methods, and many specific techniques derived from these basic methods are currently being developed to achieve convenient, non-invasive, and accurate diagnoses.
Gray scale and Doppler US are noninvasive, relatively simple, and inexpensive tests that are used to study and follow-up patients with chronic liver disease and cirrhosis. Various factors, including the liver size, bluntness of the liver edge, coarseness of the liver parenchyma, nodularity of the liver surface, portal vein (PV) velocity, and spleen size, have been suggested as useful parameters for the US-based evaluation of hepatic fibrosis or PHT in chronic liver disease[75-82]. However, although US can provide a qualitative assessment of the composition of the hepatic parenchyma, it is both subjective and operator dependent. In addition, some studies have shown that the sensitivity and specificity of US for hepatic fibrosis are unacceptably low and that there is no correlation between US findings and the histological stage of fibrosis on liver biopsy[83,84].
Regional hepatic and systemic hemodynamic changes are essential findings in liver fibrosis[85]. Therefore, Doppler US has been used to detect the hemodynamic changes that are known to be present from the pre-cirrhotic stages of hepatic fibrogenesis. Doppler US indices include the PV blood volume, mean or maximum PV velocity, portal blood flow, congestion index of the PV, effective portal liver perfusion, and resistance indices of arteries in the liver and spleen[77,85-90]. Furthermore, pulsed wave Doppler can be applied to determine the changes in the waveforms of the proper hepatic arteries, PV, and hepatic vein (HV). Although the normal flow pattern in the right HV is triphasic, patients with biphasic or monophasic flow patterns tend to have advanced fibrosis (Figure 2)[88,91-93]. In addition, HV waveform also showed promising data for the prediction of severe PHT (HVPG ≥ 12 mmHg). Especially, damping index (DI) that was calculated by the minimum velocity/maximum velocity of downward HV flow showed high accuracy with AUROC = 0.860 for the prediction of severe PHT with sensitivity 75.9% and specificity 81.8% and positive and negative predictive values 91.1% and 58.1% respectively in the value of 0.6 of DI[93]. In addition, it showed a parallel change to the change of HVPG after nonselective β blocker[93], so more studies for the ability in estimation of portal hemodynamic change are needs. However, Doppler measurement is influenced by many patient-related factors, such as respiration and the timing of meals, as well as observer variability and equipment differences. Furthermore, collateral pathways, hepatic steatosis, and inflammation further contribute to the variability in the Doppler measurements[94-96].
Taken together, gray scale and Doppler US are safe, inexpensive and simple to use at the bedside or for outpatients, and combining multiple US indices can improve the diagnostic accuracy of cirrhosis under some conditions.
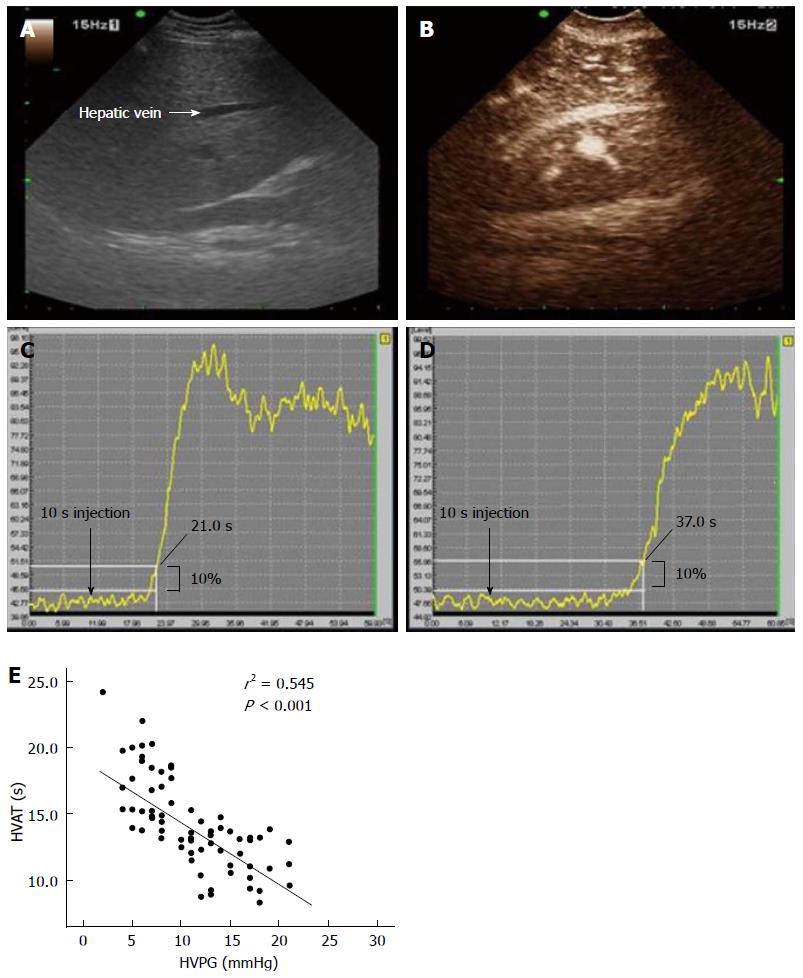
Contrast-enhanced ultrasound (CEUS) imaging represents a new US modality for the assessment of chronic liver disease. CEUS involves the intravenous administration of minute, gas-filled microbubbles that strongly enhance the intensity of signals from the intravascular flow. The various commercially available contrast agents differ in their designs and kinetics, and can therefore yield different results[97]. Bolus injections of microbubble agents can be used for first-pass kinetics studies and to assess transit times. Hepatic vein transit times (HVTT) have been shown to be reduced with worsening liver disease[98-102]. In theory, shorter hepatic vein transit times in patients with chronic liver disease mainly present secondary to arteriovenous shunting, sinusoid remodeling and arterialization of the capillary beds in the liver and, to a lesser degree, shunting in the pulmonary and gastrointestinal capillaries. In our study for compensated cirrhosis, HVTT showed a significantly strong correlation with PHT and the AUROC of HVTT for the diagnosis of CSPH was 0.973. In addition, a shorter HVTT was associated with worse Child-Pugh score (P < 0.001) and esophageal varices (P = 0.018) (Figure 3)[102]. In addition, the time intensity curve differs significantly between the normal liver parenchyma and the cirrhotic liver, and the level of decrease is related to the degree of liver damage present or PHT[102,103].
Berzigotti et al[104] reported that the regional hepatic perfusion (RHP, which was calculated as microbubbles velocity X microbubble) increased in patients with cirrhosis and correlated with the degree of liver failure. RHP increased along with liver functional reserve decrease which was measured by indocyanin green clearance and they suggested CEUS as a feasible novel, objective, quantitative, non-invasive tool for the estimation of RHP. Especially in this study, RHP showed slight positive correlation with HVPG (R = 0.279, P = 0.041) and a tendency to decrease after intravenous propranolol administration (P = 0.08). In other study[104], the signal intensity of a region of interest within the liver parenchyma have also revealed that the accumulation of microbubbles in the liver parenchyma is decreased in nonalcoholic steatohepatitis, but not in nonalcoholic fatty liver disease or chronic viral hepatitis.
CEUS-based tests for hepatic fibrosis and PHT could be simple and noninvasive tests based on contrast agent transit or the parenchymal enhancement pattern for reliably excluding cirrhosis. In particular, CEUS-based tests could be useful in the assessment of intrahepatic or systemic hemodynamic changes which are essential in advanced chronic liver disease. However, this method also has some limitations, such as the requirements for the injection of a contrast agent, considerable operator skill, and access to the relevant technology. More intensive studies and validation are needed
The increase in LS associated with chronic liver disease is primarily due to the presence of fibrosis[105]. Tissue elastography, which was introduced in 1992, is used to visualize differences in the mechanical properties among tissues[106]. The most attractive advantage of tissue elastography is its ability to quantify the viscoelasticity of the tissue, which means that it can be used to measure hepatic fibrosis in the liver. To date, two US elastography techniques have been used to measure LS: shear-wave based elastography and real-time elastography. Shear-wave based elastography includes TE (Fibroscan®), which is the most widely evaluated and used, acoustic radiation force impulse (ARFI) imaging, and supersonic shear-wave elastography (SSWE). Shear-wave based elastography involves using an ultrasonic beam to measure the propagation velocity of a shear wave through the soft tissue under investigation, and LS is displayed in kilopascals (kPa) or centimeters per second (cm/s).
TE is the first commercialized elastography protocol that was developed to noninvasively assess the stiffness of deep soft tissues such as the liver. A mechanical vibrator generates a low-frequency elastic wave at 50 Hz to produce a shear stress in the target tissue at a distance of 4 cm, after which, as mentioned above, the velocity of the shear wave is measured using an ultrasound signal. TE has been validated extensively by numerous investigations targeted at patients with chronic liver disease and cirrhosis, and TE findings are generally accepted to be strongly correlated with the stage of liver fibrosis. However, it does not provide a B-mode image which is very helpful for targeting, and it has a high measurement failure rate (15%-20%), mainly due to limiting factors such as obesity and ascites[107]. ARFI imaging and SSWE use focused high-intensity, short-duration acoustic pulses instead of the mechanical vibration used in TE to produce a shear wave in the target tissue[108]. Similar to TE, the shear-wave velocity is observed by aiming repeated ultrasound beam pulses across the region of interest (ROI). SSWE is a new type of the shear-wave based US elastography technique and uses multiple acoustic radiation force impulses[109]. It can assess the viscoelastic properties in all areas within the ROI, and the results are displayed on a color-coded lookup table. ARFI imaging and SSWE are expected to overcome the limitations of TE, such as measurement failures due to severe obesity, thick subcutaneous fat, and ascites. Moreover, they are able to display a gray scale US image on the background of the elastography and are thus more reliable and familiar to physicians who use conventional US. However, a high level of clinical experience and evidence are needed to apply either ARFI imaging or SSWE for the diagnosis of liver fibrosis.
Most clinical studies about TE have focused on its ability to identify significant fibrosis and cirrhosis, because of an indication for antiviral treatment and initiating surveillance program for the early detection of hepatocellular carcinoma (HCC) development. A number of studies have demonstrated that TE values are significantly correlated with histological fibrosis stage and have high diagnostic accuracy. In chronic hepatitis C (CHC), the AUROC of TE ranged from 0.77 to 0.90, with a cutoff value of 6.2-8.7 kPa for assessment of significant fibrosis (F ≥ 2), and an AUROC of 0.90-0.97 and cutoff value of 9.6-14.8 kPa for assessment of cirrhosis[22,110-114]. For chronic hepatitis B (CHB), AUROC and cutoff value of TE for predicting significant fibrosis and cirrhosis are 0.81-0.95, 6.3-7.9 kPa and 0.80-0.98 kPa, 9-13.8 kPa respectively[115-118]. In a recent meta-analysis, TE also showed high sensitivity and specificity in hepatic fibrosis assessment of NAFLD[119-123]. However, in case of obesity [(body mass index (BMI) ≥ 30 kg/m2] which is commonly associated in patients with NAFLD, the accuracy of TE decreases, and test may not be performable in some cases (5%-13%)[62]. Recently introduced CAP is known to relatively accurately evaluate the degree of fatty infiltration, and a large number of clinical studies are anticipated to report meaningful results[124-126].
TE also reflects a progressive rise in PHT due to increased hepatic vascular resistance related with hepatic fibrosis. TE has a good performance in discriminating between patients with and without CSPH (AUROC = 0.82-0.94)[127]. In addition, TE value < 13 kPa exclude reliably CSPH, while values > 21 kPa had an accuracy equal to that of HVPG ≥ 10 mmHg for the prediction of first clinical decompensation in patients with compensated cirrhosis[128]. As variceal bleeding is the most important complication of PHT, the relationship between TE values and the presence of EVs has been also investigated in several studies[73,129,130], and these studies showed that there is a significant correlations between TE values and the presence of EVs. However, TE still has limitations in clinical application because of the cutoff values (range, 13.9-21.5 kPa) and performance of TE variability among the studies (AUROC: 0.76-0.85)[73,129,130].
Recently a few studies have shown that the combination of TE, platelet count and spleen size by ultrasound have superior diagnostic values in the identification of the presence of CSPH and esophageal varices than single methods in patients with compensated cirrhosis of different etiologies[127]. Kim et al[131] recently proposed a novel prediction model, LS-spleen diameter to platelet ratio score (LSPS), using TE values and spleen diameter to platelet ratio that reflect PHT in patients with CHB. This model showed excellent diagnostic accuracy for prediction of high risk esophageal varices (AUROC = 0.953; negative predictive value 94.7%, positive predictive value 93.3%). Another prospective study showed that LSPS can be a reliable predictor of the development of variceal bleeding. In this prospective, CHB patients with LSPS ≥ 5.5 had higher cumulative incidence rates of esophageal variceal bleeding during the follow-up period and LSPS score ≥ 6.5 was an independent risk factor of variceal bleeding from high risk esophageal varices, indicating that prophylactic treatment should be considered in these high risk patients[132]. A recent validation study for LSPS showed that more than 80% of patients were accurately classified using LSPS. Additionally, in this study, a new modified LSPS, varices risk score were suggested and it was superior to all other noninvasive tests for identifying patients with EVs (AUROC = 0.909) with 85% correct classification of patients[133].
ARFI imaging has similar accuracy with TE or ELF for significant fibrosis with AUROC = 0.879 (0.861 and 0.764, for TE and ELF, respectively) and cirrhosis (0.936, 0.918, and 0.841) and the combination of ELF with ARFI imaging or TE increased the negative and positive predictive values of single tests for the diagnosis of significant fibrosis and cirrhosis[134]. A recent meta-analysis included 13 studies in which 1163 patients with chronic liver diseases showed similar sensitivity and specificity between ARFI imaging and TE for detection of significant fibrosis (≥ F2) (Se and Sp were 0.74 and 0.83 for ARFI imaging, 0.78 and 0.84 for TE) and cirrhosis (0.87 and 0.87 for ARFI imaging, 0.89 and 0.87 for TE)[135]. For the relationship with PHT, just a little data can be found and ARFI imaging also showed a good correlation with HVPG (R = 0.709) and AUROC = 0.874 for predicting CSPH[136]. However, in the prediction of EVs, ARFI imaging just has shown low AUROC = 0.58 and more data is needed for establishment of its clinical significance[137].
In case of SSWE, one study evaluated 133 patients with chronic hepatitis C by means of SSWE, TE and, in a subgroup of patients, with liver biopsy. The AUROCs of SSEW were 0.948 for ≥ F2, 0.962 for ≥ F3 and 0.968 for F4 cirrhosis[138]. Because just a little data has been reported, more data is needed for establishment of its clinical usefulness.
Another new trial in this field is to measurement of spleen stiffness (SS). As well known, spleen congestion is a specific feature of PHT; SS using TE or ARFI imaging has been recently applied to diagnose of CSPH and esophageal varices, and had a higher accuracy than other noninvasive parameters such as LSPS or platelet/spleen ratio and MR elastography (MRE)[132,133,139]. In a recent study, SS using TE showed AUROC = 0.941, 0.966 and 0.957 for the prediction of EV, CSPH and severe PHT (HVPG ≥ 12 mmHg) respectively and these were similar with LS using TE[139]. In case of SS using ARFI, SS showed better accuracy in the prediction of EV than LS (AUROC = 0.933 vs 0.746)[140]. In addition, in a study for 65 patients with EHPVO, LS and SS were higher in patients with EHPVO (6.7 ± 2.3 kPa and 51.7 ± 21.5 kPa, respectively) than in control subjects (4.6 ± 0.7 kPa and 16.0 ± 3.0 kPa, respectively) and patients with a history of a bleed had a higher SS than did those without a bleed(60.4 ± 5.4 kPa vs 30.3 ± 14.2 kPa, P = 0.01). So, SS showed a possibility as a predictor for variceal hemorrhage in this disease population[141]. However, measurement of SS with TE could have substantial limitations in real performance and reproducibility, more validation has to be followed.
Because advanced liver fibrosis and cirrhosis are the most important risk factors of HCC development, several studies have evaluated the clinical role of TE for predicting HCC development[142,143]. In CHC patients study, patients with higher TE values had a significantly higher risk of HCC development according to the increase of TE values (hazard ratio of 16.7 with 10.1-15 kPa, 20.9 with 15.1-20 kPa, 25.6 with 20.1-25 kPa, 45.5 with over 25 kPa) as compared to under 10 kPa[142]. Similarly, in populations with CHB, TE value was also identified as an independent risk factor for HCC development, with relative risks of 3.07, 4.68, 5.55, and 6.60 for respective TE values of 8-13 kPa, 13-18 kPa, 18-23 kPa, and > 23 kPa compared TE value to less than 8 kPa[143].
In summary, the development of various promising techniques for measuring LS have helped overcome the limitations not only for evaluating hepatic fibrosis or CSPH but also for predicting the development of fatal complications related to chronic liver disease and the patient’s prognosis. Therefore, they can be considered a useful tool to reduce the need of HVPG measurement and endoscopy, and to improve the selection of patients requiring further risk stratification. However, despite the above mentioned advances in non-invasive assessment of PHT, HVPG measurement cannot be fully replaced. In addition, they cannot accurately predict the hemodynamic response to non-selective β blocker[144]. Further investigations in this field have to be followed.
Cross-sectional imaging studies such as CT and MRI are useful imaging modalities for the diagnosis of cirrhosis. These modalities are considered to be standard methods for the diagnosis of HCC on the background of chronic liver disease, including cirrhosis[139,140,145]. The radiologic features of advanced cirrhosis are normally obvious and include surface nodularity, prominent fibrous septa, shrinkage of liver volume, and an enlarged portal venous system including varices and splenomegaly due to PHT. However, it is difficult to diagnose the early stage of cirrhosis. As such, various functional techniques using CT and MRI have been developed recently and described in many hepatology and radiology journals.
Morphologic changes: In the advanced stages of cirrhosis, the morphologic changes in the liver can be clearly demonstrated by both CT and MRI. Although the imaging features are highly specific for cirrhosis, the sensitivity is very low for early cirrhosis. As cross-sectional imaging can be used for the diagnosis of cirrhosis, several imaging signs related to morphologic changes of the liver have been investigated. A modified caudate: right-lobe ratio is one of these morphologic CT signs[146,147], and is defined by using the bifurcation of the right PV as a landmark, which is considered to be a lateral boundary for the caudate lobe and a medial boundary for the right lobe. According to that study, the sensitivity and accuracy for diagnosing cirrhosis was approximately 72% and 74%, respectively, when using a modified caudate: right-lobe ratio cut-off of greater than 0.90. The use of these signs produces a very high specificity and positive predictive value for diagnosing cirrhosis, but usually a low sensitivity. In other words, the morphologic changes associated with cirrhosis that is visible on CT and MRI is usually only observed in advanced cases. As such, CT and MRI are not suitable for the early diagnosis of cirrhosis.
Hemodynamic changes: Hemodynamically, the cirrhotic liver is highly resistant to portal inflow, and the systemic response that increase the splanchnic circulation aggravates the situation, creating a vicious cycle that may result in the development of PHT, hyperdynamic circulation and its complications[148]. Because conventional CT and MRI are not functional imaging modalities, they are not appropriate for evaluating the hemodynamic changes in the liver. However, they are useful for observing the secondary changes of PHT in a cirrhotic patient, such as portosystemic collateral formation, splenomegaly, portal hypertensive gastropathy, portal hypertensive colopathy, and ascites.
Esophageal and paraesophageal varices are common complications of cirrhosis, and arise from the impaired venous drainage of the esophageal vein due to increased portal venous pressure. A gastric varix is less common than an esophageal varix and occurs in approximately 20% of patients with PHT[149]. In general, the estimation of the risk of variceal bleeding is made by using endoscopic findings such as the size, color, and location of varices[150]. However, in the era of multidetector CT, which enables CT scanning at a submillimeter thickness, CT can be used to obtain information not only about the cirrhotic liver itself but also about the PHT caused by cirrhosis. Various portosystemic collateral veins can also be depicted in the CT scan, and physicians can plan a strategy for the treatment of varices, including the insertion of a transjugular intrahepatic portosystemic shunt and balloon-occluded retrograde transvenous obliteration. Moreover, as with endoscopy, demonstrating the presence of esophageal and gastric varices is now possible using CT[151]. The sensitivity and specificity of CT were found to be 96% and 55%, respectively, to detect esophageal varices and 93% and 80%, respectively to detect high-risk esophageal varices[152]. Using the 1- to 3-mm multiplanar reformat or surface-shaded display can also increase the specificity of CT for the risk stratification of esophageal varices101. With regard to gastric varices, the sensitivity and specificity were 83%-89% and 75%-79%, respectively[153,154]. Although these results are not bad, the accuracy for small varices remains low.
MRE: MRE is an emerging technology that is used to quantitatively assess the elasticity of the liver[86]. This modality can improve the contrast between different tissues in the body compared with other imaging modalities, such as ultrasound, CT, and conventional MRI. MRE has been used in clinical practice for the assessment of hepatic fibrosis since 2006[155,156]. Several clinical studies have established the feasibility of MRE for the evaluation of hepatic fibrosis[156-160], and in a recent meta-analysis, the overall sensitivity, specificity, and AUROC of MRE for histological grade ≥ F2 were 0.94, 0.95, and 0.98 and for ≥ F4 were 0.99, 0.94, and 0.99 respectively[161].
There are several advantages of MRE over US based elastography[158,166], such as not being influenced by body habitus, not being operator dependent, and its potential ability to assess the entire liver. However, this technique is expensive and cannot yet be regularly used in all medical institutions and further research is required to validate liver MRE against long-term clinical outcomes.
Chronic liver disease is a heterogeneous and dynamic condition. The exact estimations of the stage and the changes in hepatic fibrosis and PHT are essential in the management of patients with this disease. Until now, no non-invasive diagnostic tests have satisfied such clinical needs. Recently, a few tests, such as LS measurement based on TE or MR and CEUS-based hemodynamic analysis, have shown promising results in this field[167]. However, the reproducibility of these non-invasive tests needs to be validated in diverse clinical situations and etiologies. Especially, it is important to study about the long term prognostic value non-invasive tests in hepatic fibrosis and PHT that can leads to new paradigm in the tailored management of chronic liver disease. Notably, the integration of information derived from serologic tests with that of other non-invasive tests, such as TE, allows the progression in the management of patients with chronic liver disease in the future.
P- Reviewers: Berzigotti A, Procopet B, Sharma V S- Editor: Qi Y L- Editor: A E- Editor: Ma S
| 1. | Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF, Schall RA. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1228] [Cited by in F6Publishing: 1226] [Article Influence: 111.5] [Reference Citation Analysis (0)] |
| 2. | Schalm SW. The diagnosis of cirrhosis: clinical relevance and methodology. J Hepatol. 1997;27:1118-1119. [PubMed] [Cited in This Article: ] |
| 3. | Friedman SL, Bansal MB. Reversal of hepatic fibrosis -- fact or fantasy? Hepatology. 2006;43:S82-S88. [PubMed] [Cited in This Article: ] |
| 4. | Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495-500. [PubMed] [Cited in This Article: ] |
| 5. | Bosch J, Abraldes JG, Berzigotti A, García-Pagan JC. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol. 2009;6:573-582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 451] [Cited by in F6Publishing: 454] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 6. | Bonekamp S, Kamel I, Solga S, Clark J. Can imaging modalities diagnose and stage hepatic fibrosis and cirrhosis accurately? J Hepatol. 2009;50:17-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 7. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [PubMed] [Cited in This Article: ] |
| 8. | Kim MY, Cho MY, Baik SK, Park HJ, Jeon HK, Im CK, Won CS, Kim JW, Kim HS, Kwon SO. Histological subclassification of cirrhosis using the Laennec fibrosis scoring system correlates with clinical stage and grade of portal hypertension. J Hepatol. 2011;55:1004-1009. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 9. | Ludwig J. The nomenclature of chronic active hepatitis: an obituary. Gastroenterology. 1993;105:274-278. [PubMed] [Cited in This Article: ] |
| 10. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [PubMed] [Cited in This Article: ] |
| 11. | Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13:372-374. [PubMed] [Cited in This Article: ] |
| 12. | Hytiroglou P, Thung SN, Gerber MA. Histological classification and quantitation of the severity of chronic hepatitis: keep it simple! Semin Liver Dis. 1995;15:414-421. [PubMed] [Cited in This Article: ] |
| 13. | Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol. 2003;39:239-244. [PubMed] [Cited in This Article: ] |
| 14. | Afdhal NH, Nunes D. Evaluation of liver fibrosis: a concise review. Am J Gastroenterol. 2004;99:1160-1174. [PubMed] [Cited in This Article: ] |
| 15. | D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231. [PubMed] [Cited in This Article: ] |
| 16. | Garcia-Tsao G, Friedman S, Iredale J, Pinzani M. Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology. 2010;51:1445-1449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 359] [Cited by in F6Publishing: 361] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 17. | Kim SU, Oh HJ, Wanless IR, Lee S, Han KH, Park YN. The Laennec staging system for histological sub-classification of cirrhosis is useful for stratification of prognosis in patients with liver cirrhosis. J Hepatol. 2012;57:556-563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 18. | Nagula S, Jain D, Groszmann RJ, Garcia-Tsao G. Histological-hemodynamic correlation in cirrhosis-a histological classification of the severity of cirrhosis. J Hepatol. 2006;44:111-117. [PubMed] [Cited in This Article: ] |
| 19. | Kumar M, Sakhuja P, Kumar A, Manglik N, Choudhury A, Hissar S, Rastogi A, Sarin SK. Histological subclassification of cirrhosis based on histological-haemodynamic correlation. Aliment Pharmacol Ther. 2008;27:771-779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449-1457. [PubMed] [Cited in This Article: ] |
| 21. | Calvaruso V, Burroughs AK, Standish R, Manousou P, Grillo F, Leandro G, Maimone S, Pleguezuelo M, Xirouchakis I, Guerrini GP. Computer-assisted image analysis of liver collagen: relationship to Ishak scoring and hepatic venous pressure gradient. Hepatology. 2009;49:1236-1244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 213] [Cited by in F6Publishing: 199] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 22. | Nitta Y, Kawabe N, Hashimoto S, Harata M, Komura N, Kobayashi K, Arima Y, Shimazaki H, Nakano T, Murao M. Liver stiffness measured by transient elastography correlates with fibrosis area in liver biopsy in patients with chronic hepatitis C. Hepatol Res. 2009;39:675-684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Ziol M, Kettaneh A, Ganne-Carrié N, Barget N, Tengher-Barna I, Beaugrand M. Relationships between fibrosis amounts assessed by morphometry and liver stiffness measurements in chronic hepatitis or steatohepatitis. Eur J Gastroenterol Hepatol. 2009;21:1261-1268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Perelló A, Escorsell A, Bru C, Gilabert R, Moitinho E, García-Pagán JC, Bosch J. Wedged hepatic venous pressure adequately reflects portal pressure in hepatitis C virus-related cirrhosis. Hepatology. 1999;30:1393-1397. [PubMed] [Cited in This Article: ] |
| 25. | Ripoll C, Groszmann R, Garcia-Tsao G, Grace N, Burroughs A, Planas R, Escorsell A, Garcia-Pagan JC, Makuch R, Patch D. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481-488. [PubMed] [Cited in This Article: ] |
| 26. | Lebrec D, De Fleury P, Rueff B, Nahum H, Benhamou JP. Portal hypertension, size of esophageal varices, and risk of gastrointestinal bleeding in alcoholic cirrhosis. Gastroenterology. 1980;79:1139-1144. [PubMed] [Cited in This Article: ] |
| 27. | Groszmann RJ, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Planas R, Escorsell A, Garcia-Pagan JC, Patch D, Matloff DS. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353:2254-2261. [PubMed] [Cited in This Article: ] |
| 28. | Ripoll C, Bañares R, Rincón D, Catalina MV, Lo Iacono O, Salcedo M, Clemente G, Núñez O, Matilla A, Molinero LM. Influence of hepatic venous pressure gradient on the prediction of survival of patients with cirrhosis in the MELD Era. Hepatology. 2005;42:793-801. [PubMed] [Cited in This Article: ] |
| 29. | Burroughs AK, Groszmann R, Bosch J, Grace N, Garcia-Tsao G, Patch D, Garcia-Pagan JC, Dagher L. Assessment of therapeutic benefit of antiviral therapy in chronic hepatitis C: is hepatic venous pressure gradient a better end point? Gut. 2002;50:425-427. [PubMed] [Cited in This Article: ] |
| 30. | Kumar M, Kumar A, Hissar S, Jain P, Rastogi A, Kumar D, Sakhuja P, Sarin SK. Hepatic venous pressure gradient as a predictor of fibrosis in chronic liver disease because of hepatitis B virus. Liver Int. 2008;28:690-698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Rincon D, Ripoll C, Lo Iacono O, Salcedo M, Catalina MV, Alvarez E, Nuñez O, Matilla AM, Clemente G, Bañares R. Antiviral therapy decreases hepatic venous pressure gradient in patients with chronic hepatitis C and advanced fibrosis. Am J Gastroenterol. 2006;101:2269-2274. [PubMed] [Cited in This Article: ] |
| 32. | Roberts S, Gordon A, McLean C, Pedersen J, Bowden S, Thomson K, Angus P. Effect of sustained viral response on hepatic venous pressure gradient in hepatitis C-related cirrhosis. Clin Gastroenterol Hepatol. 2007;5:932-937. [PubMed] [Cited in This Article: ] |
| 33. | Blasco A, Forns X, Carrión JA, García-Pagán JC, Gilabert R, Rimola A, Miquel R, Bruguera M, García-Valdecasas JC, Bosch J. Hepatic venous pressure gradient identifies patients at risk of severe hepatitis C recurrence after liver transplantation. Hepatology. 2006;43:492-499. [PubMed] [Cited in This Article: ] |
| 34. | Carrión JA, Navasa M, Bosch J, Bruguera M, Gilabert R, Forns X. Transient elastography for diagnosis of advanced fibrosis and portal hypertension in patients with hepatitis C recurrence after liver transplantation. Liver Transpl. 2006;12:1791-1798. [PubMed] [Cited in This Article: ] |
| 35. | Samonakis DN, Cholongitas E, Thalheimer U, Kalambokis G, Quaglia A, Triantos CK, Mela M, Manousou P, Senzolo M, Dhillon AP. Hepatic venous pressure gradient to assess fibrosis and its progression after liver transplantation for HCV cirrhosis. Liver Transpl. 2007;13:1305-1311. [PubMed] [Cited in This Article: ] |
| 36. | Ripoll C, Groszmann RJ, Garcia-Tsao G, Bosch J, Grace N, Burroughs A, Planas R, Escorsell A, Garcia-Pagan JC, Makuch R. Hepatic venous pressure gradient predicts development of hepatocellular carcinoma independently of severity of cirrhosis. J Hepatol. 2009;50:923-928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 284] [Cited by in F6Publishing: 264] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 37. | Bruix J, Castells A, Bosch J, Feu F, Fuster J, Garcia-Pagan JC, Visa J, Bru C, Rodés J. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology. 1996;111:1018-1022. [PubMed] [Cited in This Article: ] |
| 38. | Forner A, Bruix J. East meets the West--portal pressure predicts outcome of surgical resection for hepatocellular carcinoma. Nat Clin Pract Gastroenterol Hepatol. 2009;6:14-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Khanna R, Sarin SK. Non-cirrhotic portal hypertension - diagnosis and management. J Hepatol. 2014;60:421-441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 211] [Article Influence: 21.1] [Reference Citation Analysis (2)] |
| 40. | Garcia-Tsao G, Groszmann RJ, Fisher RL, Conn HO, Atterbury CE, Glickman M. Portal pressure, presence of gastroesophageal varices and variceal bleeding. Hepatology. 1985;5:419-424. [PubMed] [Cited in This Article: ] |
| 41. | Feu F, García-Pagán JC, Bosch J, Luca A, Terés J, Escorsell A, Rodés J. Relation between portal pressure response to pharmacotherapy and risk of recurrent variceal haemorrhage in patients with cirrhosis. Lancet. 1995;346:1056-1059. [PubMed] [Cited in This Article: ] |
| 42. | D'Amico G, Garcia-Pagan JC, Luca A, Bosch J. Hepatic vein pressure gradient reduction and prevention of variceal bleeding in cirrhosis: a systematic review. Gastroenterology. 2006;131:1611-1624. [PubMed] [Cited in This Article: ] |
| 43. | Abraldes JG, Tarantino I, Turnes J, Garcia-Pagan JC, Rodés J, Bosch J. Hemodynamic response to pharmacological treatment of portal hypertension and long-term prognosis of cirrhosis. Hepatology. 2003;37:902-908. [PubMed] [Cited in This Article: ] |
| 44. | Villanueva C, López-Balaguer JM, Aracil C, Kolle L, González B, Miñana J, Soriano G, Guarner C, Balanzó J. Maintenance of hemodynamic response to treatment for portal hypertension and influence on complications of cirrhosis. J Hepatol. 2004;40:757-765. [PubMed] [Cited in This Article: ] |
| 45. | La Mura V, Abraldes JG, Raffa S, Retto O, Berzigotti A, García-Pagán JC, Bosch J. Prognostic value of acute hemodynamic response to i.v. propranolol in patients with cirrhosis and portal hypertension. J Hepatol. 2009;51:279-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 46. | Villanueva C, Aracil C, Colomo A, Hernández-Gea V, López-Balaguer JM, Alvarez-Urturi C, Torras X, Balanzó J, Guarner C. Acute hemodynamic response to beta-blockers and prediction of long-term outcome in primary prophylaxis of variceal bleeding. Gastroenterology. 2009;137:119-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 160] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 47. | de Franchis R, Dell’Era A, Primignani M. Diagnosis and monitoring of portal hypertension. Dig Liver Dis. 2008;40:312-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | Vizzutti F, Arena U, Rega L, Pinzani M. Non invasive diagnosis of portal hypertension in cirrhotic patients. Gastroenterol Clin Biol. 2008;32:80-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 49. | Berzigotti A, Ashkenazi E, Reverter E, Abraldes JG, Bosch J. Non-invasive diagnostic and prognostic evaluation of liver cirrhosis and portal hypertension. Dis Markers. 2011;31:129-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 22] [Reference Citation Analysis (0)] |
| 50. | Leroy V, Hilleret MN, Sturm N, Trocme C, Renversez JC, Faure P, Morel F, Zarski JP. Prospective comparison of six non-invasive scores for the diagnosis of liver fibrosis in chronic hepatitis C. J Hepatol. 2007;46:775-782. [PubMed] [Cited in This Article: ] |
| 51. | Sebastiani G, Alberti A. Non invasive fibrosis biomarkers reduce but not substitute the need for liver biopsy. World J Gastroenterol. 2006;12:3682-3694. [PubMed] [Cited in This Article: ] |
| 52. | Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069-1075. [PubMed] [Cited in This Article: ] |
| 53. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [PubMed] [Cited in This Article: ] |
| 54. | Parkes J, Guha IN, Roderick P, Rosenberg W. Performance of serum marker panels for liver fibrosis in chronic hepatitis C. J Hepatol. 2006;44:462-474. [PubMed] [Cited in This Article: ] |
| 55. | Gebo KA, Herlong HF, Torbenson MS, Jenckes MW, Chander G, Ghanem KG, El-Kamary SS, Sulkowski M, Bass EB. Role of liver biopsy in management of chronic hepatitis C: a systematic review. Hepatology. 2002;36:S161-S172. [PubMed] [Cited in This Article: ] |
| 56. | Rosenberg WM, Voelker M, Thiel R, Becka M, Burt A, Schuppan D, Hubscher S, Roskams T, Pinzani M, Arthur MJ. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004;127:1704-1713. [PubMed] [Cited in This Article: ] |
| 57. | Parkes J, Guha IN, Roderick P, Harris S, Cross R, Manos MM, Irving W, Zaitoun A, Wheatley M, Ryder S. Enhanced Liver Fibrosis (ELF) test accurately identifies liver fibrosis in patients with chronic hepatitis C. J Viral Hepat. 2011;18:23-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 58. | Guha IN, Parkes J, Roderick P, Chattopadhyay D, Cross R, Harris S, Kaye P, Burt AD, Ryder SD, Aithal GP. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: Validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology. 2008;47:455-460. [PubMed] [Cited in This Article: ] |
| 59. | Mayo MJ, Parkes J, Adams-Huet B, Combes B, Mills AS, Markin RS, Rubin R, Wheeler D, Contos M, West AB. Prediction of clinical outcomes in primary biliary cirrhosis by serum enhanced liver fibrosis assay. Hepatology. 2008;48:1549-1557. [PubMed] [Cited in This Article: ] |
| 60. | Nobili V, Parkes J, Bottazzo G, Marcellini M, Cross R, Newman D, Vizzutti F, Pinzani M, Rosenberg WM. Performance of ELF serum markers in predicting fibrosis stage in pediatric non-alcoholic fatty liver disease. Gastroenterology. 2009;136:160-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 190] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 61. | Parkes J, Roderick P, Harris S, Day C, Mutimer D, Collier J, Lombard M, Alexander G, Ramage J, Dusheiko G. Enhanced liver fibrosis test can predict clinical outcomes in patients with chronic liver disease. Gut. 2010;59:1245-1251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 228] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 62. | Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 886] [Cited by in F6Publishing: 854] [Article Influence: 65.7] [Reference Citation Analysis (0)] |
| 63. | Wieckowska A, McCullough AJ, Feldstein AE. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: present and future. Hepatology. 2007;46:582-589. [PubMed] [Cited in This Article: ] |
| 64. | Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44:27-33. [PubMed] [Cited in This Article: ] |
| 65. | Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846-854. [PubMed] [Cited in This Article: ] |
| 66. | Gressner AM, Tittor W, Negwer A, Pick-Kober KH. Serum concentrations of laminin and aminoterminal propeptide of type III procollagen in relation to the portal venous pressure of fibrotic liver diseases. Clin Chim Acta. 1986;161:249-258. [PubMed] [Cited in This Article: ] |
| 67. | Kondo M, Miszputen SJ, Leite-mor MM, Parise ER. The predictive value of serum laminin for the risk of variceal bleeding related to portal pressure levels. Hepatogastroenterology. 1995;42:542-545. [PubMed] [Cited in This Article: ] |
| 68. | Kropf J, Gressner AM, Tittor W. Logistic-regression model for assessing portal hypertension by measuring hyaluronic acid (hyaluronan) and laminin in serum. Clin Chem. 1991;37:30-35. [PubMed] [Cited in This Article: ] |
| 69. | Thabut D, Imbert-Bismut F, Cazals-Hatem D, Messous D, Muntenau M, Valla DC, Moreau R, Poynard T, Lebrec D. Relationship between the Fibrotest and portal hypertension in patients with liver disease. Aliment Pharmacol Ther. 2007;26:359-368. [PubMed] [Cited in This Article: ] |
| 70. | Schepis F, Cammà C, Niceforo D, Magnano A, Pallio S, Cinquegrani M, D’amico G, Pasta L, Craxì A, Saitta A. Which patients with cirrhosis should undergo endoscopic screening for esophageal varices detection? Hepatology. 2001;33:333-338. [PubMed] [Cited in This Article: ] |
| 71. | Zaman A, Hapke R, Flora K, Rosen HR, Benner K. Factors predicting the presence of esophageal or gastric varices in patients with advanced liver disease. Am J Gastroenterol. 1999;94:3292-3296. [PubMed] [Cited in This Article: ] |
| 72. | Giannini E, Botta F, Borro P, Risso D, Romagnoli P, Fasoli A, Mele MR, Testa E, Mansi C, Savarino V. Platelet count/spleen diameter ratio: proposal and validation of a non-invasive parameter to predict the presence of oesophageal varices in patients with liver cirrhosis. Gut. 2003;52:1200-1205. [PubMed] [Cited in This Article: ] |
| 73. | Castéra L, Le Bail B, Roudot-Thoraval F, Bernard PH, Foucher J, Merrouche W, Couzigou P, de Lédinghen V. Early detection in routine clinical practice of cirrhosis and oesophageal varices in chronic hepatitis C: comparison of transient elastography (FibroScan) with standard laboratory tests and non-invasive scores. J Hepatol. 2009;50:59-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 272] [Cited by in F6Publishing: 287] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 74. | Castéra L, Sebastiani G, Le Bail B, de Lédinghen V, Couzigou P, Alberti A. Prospective comparison of two algorithms combining non-invasive methods for staging liver fibrosis in chronic hepatitis C. J Hepatol. 2010;52:191-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 152] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 75. | Colli A, Fraquelli M, Andreoletti M, Marino B, Zuccoli E, Conte D. Severe liver fibrosis or cirrhosis: accuracy of US for detection--analysis of 300 cases. Radiology. 2003;227:89-94. [PubMed] [Cited in This Article: ] |
| 76. | Oberti F, Valsesia E, Pilette C, Rousselet MC, Bedossa P, Aubé C, Gallois Y, Rifflet H, Maïga MY, Penneau-Fontbonne D. Noninvasive diagnosis of hepatic fibrosis or cirrhosis. Gastroenterology. 1997;113:1609-1616. [PubMed] [Cited in This Article: ] |
| 77. | Aubé C, Oberti F, Korali N, Namour MA, Loisel D, Tanguy JY, Valsesia E, Pilette C, Rousselet MC, Bedossa P. Ultrasonographic diagnosis of hepatic fibrosis or cirrhosis. J Hepatol. 1999;30:472-478. [PubMed] [Cited in This Article: ] |
| 78. | Shen L, Li JQ, Zeng MD, Lu LG, Fan ST, Bao H. Correlation between ultrasonographic and pathologic diagnosis of liver fibrosis due to chronic virus hepatitis. World J Gastroenterol. 2006;12:1292-1295. [PubMed] [Cited in This Article: ] |
| 79. | Celle G, Savarino V, Picciotto A, Magnolia MR, Scalabrini P, Dodero M. Is hepatic ultrasonography a valid alternative tool to liver biopsy? Report on 507 cases studied with both techniques. Dig Dis Sci. 1988;33:467-471. [PubMed] [Cited in This Article: ] |
| 80. | Gaiani S, Gramantieri L, Venturoli N, Piscaglia F, Siringo S, D’Errico A, Zironi G, Grigioni W, Bolondi L. What is the criterion for differentiating chronic hepatitis from compensated cirrhosis? A prospective study comparing ultrasonography and percutaneous liver biopsy. J Hepatol. 1997;27:979-985. [PubMed] [Cited in This Article: ] |
| 81. | Khan KN, Yamasaki M, Yamasaki K, Inoue O, Yatsuhashi H, Koga M, Yano M. Proposed abdominal sonographic staging to predict severity of liver diseases: analysis with peritoneoscopy and histology. Dig Dis Sci. 2000;45:554-564. [PubMed] [Cited in This Article: ] |
| 82. | Nicolau C, Bianchi L, Vilana R. Gray-scale ultrasound in hepatic cirrhosis and chronic hepatitis: diagnosis, screening, and intervention. Semin Ultrasound CT MR. 2002;23:3-18. [PubMed] [Cited in This Article: ] |
| 83. | Chen CH, Lin ST, Yang CC, Yeh YH, Kuo CL, Nien CK. The accuracy of sonography in predicting steatosis and fibrosis in chronic hepatitis C. Dig Dis Sci. 2008;53:1699-1706. [PubMed] [Cited in This Article: ] |
| 84. | Kutcher R, Smith GS, Sen F, Gelman SF, Mitsudo S, Thung SN, Reinus JF. Comparison of sonograms and liver histologic findings in patients with chronic hepatitis C virus infection. J Ultrasound Med. 1998;17:321-325. [PubMed] [Cited in This Article: ] |
| 85. | Baik SK. Haemodynamic evaluation by Doppler ultrasonography in patients with portal hypertension: a review. Liver Int. 2010;30:1403-1413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 86. | Bolognesi M, Sacerdoti D, Mescoli C, Bombonato G, Cillo U, Merenda R, Giacomelli L, Merkel C, Rugge M, Gatta A. Different hemodynamic patterns of alcoholic and viral endstage cirrhosis: analysis of explanted liver weight, degree of fibrosis and splanchnic Doppler parameters. Scand J Gastroenterol. 2007;42:256-262. [PubMed] [Cited in This Article: ] |
| 87. | Iliopoulos P, Vlychou M, Margaritis V, Tsamis I, Tepetes K, Petsas T, Karatza C. Gray and color Doppler ultrasonography in differentiation between chronic viral hepatitis and compensated early stage cirrhosis. J Gastrointestin Liver Dis. 2007;16:279-286. [PubMed] [Cited in This Article: ] |
| 88. | Haktanir A, Cihan BS, Celenk C, Cihan S. Value of Doppler sonography in assessing the progression of chronic viral hepatitis and in the diagnosis and grading of cirrhosis. J Ultrasound Med. 2005;24:311-321. [PubMed] [Cited in This Article: ] |
| 89. | Iwao T, Toyonaga A, Oho K, Tayama C, Masumoto H, Sakai T, Sato M, Tanikawa K. Value of Doppler ultrasound parameters of portal vein and hepatic artery in the diagnosis of cirrhosis and portal hypertension. Am J Gastroenterol. 1997;92:1012-1017. [PubMed] [Cited in This Article: ] |
| 90. | Liu CH, Lin JW, Tsai FC, Yang PM, Lai MY, Chen JH, Kao JH, Chen DS. Noninvasive tests for the prediction of significant hepatic fibrosis in hepatitis C virus carriers with persistently normal alanine aminotransferases. Liver Int. 2006;26:1087-1094. [PubMed] [Cited in This Article: ] |
| 91. | Arda K, Ofelli M, Calikoglu U, Olçer T, Cumhur T. Hepatic vein Doppler waveform changes in early stage (Child-Pugh A) chronic parenchymal liver disease. J Clin Ultrasound. 1997;25:15-19. [PubMed] [Cited in This Article: ] |
| 92. | Baik SK, Kim JW, Kim HS, Kwon SO, Kim YJ, Park JW, Kim SH, Chang SJ, Lee DK, Han KH. Recent variceal bleeding: Doppler US hepatic vein waveform in assessment of severity of portal hypertension and vasoactive drug response. Radiology. 2006;240:574-580. [PubMed] [Cited in This Article: ] |
| 93. | Kim MY, Baik SK, Park DH, Lim DW, Kim JW, Kim HS, Kwon SO, Kim YJ, Chang SJ, Lee SS. Damping index of Doppler hepatic vein waveform to assess the severity of portal hypertension and response to propranolol in liver cirrhosis: a prospective nonrandomized study. Liver Int. 2007;27:1103-1110. [PubMed] [Cited in This Article: ] |
| 94. | Bernatik T, Strobel D, Hahn EG, Becker D. Doppler measurements: a surrogate marker of liver fibrosis? Eur J Gastroenterol Hepatol. 2002;14:383-387. [PubMed] [Cited in This Article: ] |
| 95. | Lim AK, Patel N, Eckersley RJ, Kuo YT, Goldin RD, Thomas HC, Cosgrove DO, Taylor-Robinson SD, Blomley MJ. Can Doppler sonography grade the severity of hepatitis C-related liver disease? AJR Am J Roentgenol. 2005;184:1848-1853. [PubMed] [Cited in This Article: ] |
| 96. | Colli A, Cocciolo M, Mumoli N, Cattalini N, Fraquelli M, Conte D. Hepatic artery resistance in alcoholic liver disease. Hepatology. 1998;28:1182-1186. [PubMed] [Cited in This Article: ] |
| 97. | Klibanov AL. Ultrasound molecular imaging with targeted microbubble contrast agents. J Nucl Cardiol. 2007;14:876-884. [PubMed] [Cited in This Article: ] |
| 98. | Albrecht T, Blomley MJ, Cosgrove DO, Taylor-Robinson SD, Jayaram V, Eckersley R, Urbank A, Butler-Barnes J, Patel N. Non-invasive diagnosis of hepatic cirrhosis by transit-time analysis of an ultrasound contrast agent. Lancet. 1999;353:1579-1583. [PubMed] [Cited in This Article: ] |
| 99. | Blomley MJ, Lim AK, Harvey CJ, Patel N, Eckersley RJ, Basilico R, Heckemann R, Urbank A, Cosgrove DO, Taylor-Robinson SD. Liver microbubble transit time compared with histology and Child-Pugh score in diffuse liver disease: a cross sectional study. Gut. 2003;52:1188-1193. [PubMed] [Cited in This Article: ] |
| 100. | Lim AK, Taylor-Robinson SD, Patel N, Eckersley RJ, Goldin RD, Hamilton G, Foster GR, Thomas HC, Cosgrove DO, Blomley MJ. Hepatic vein transit times using a microbubble agent can predict disease severity non-invasively in patients with hepatitis C. Gut. 2005;54:128-133. [PubMed] [Cited in This Article: ] |
| 101. | Lim AK, Patel N, Eckersley RJ, Goldin RD, Thomas HC, Cosgrove DO, Taylor-Robinson SD, Blomley MJ. Hepatic vein transit time of SonoVue: a comparative study with Levovist. Radiology. 2006;240:130-135. [PubMed] [Cited in This Article: ] |
| 102. | Kim MY, Suk KT, Baik SK, Kim HA, Kim YJ, Cha SH, Kwak HR, Cho MY, Park HJ, Jeon HK. Hepatic vein arrival time as assessed by contrast-enhanced ultrasonography is useful for the assessment of portal hypertension in compensated cirrhosis. Hepatology. 2012;56:1053-1062. [PubMed] [Cited in This Article: ] |
| 103. | Kaneko T, Teshigawara O, Sugimoto H, Hirota M, Inoue S, Takeda S, Nakao A. Signal intensity of the liver parenchyma in microbubble contrast agent in the late liver phase reflects advanced fibrosis of the liver. Liver Int. 2005;25:288-293. [PubMed] [Cited in This Article: ] |
| 104. | Berzigotti A, Nicolau C, Bellot P, Abraldes JG, Gilabert R, García-Pagan JC, Bosch J. Evaluation of regional hepatic perfusion (RHP) by contrast-enhanced ultrasound in patients with cirrhosis. J Hepatol. 2011;55:307-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 105. | Iijima H, Moriyasu F, Tsuchiya K, Suzuki S, Yoshida M, Shimizu M, Sasaki S, Nishiguchi S, Maeyama S. Decrease in accumulation of ultrasound contrast microbubbles in non-alcoholic steatohepatitis. Hepatol Res. 2007;37:722-730. [PubMed] [Cited in This Article: ] |
| 106. | Talwalkar JA. Elastography for detecting hepatic fibrosis: options and considerations. Gastroenterology. 2008;135:299-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 107. | Ophir J, Céspedes I, Ponnekanti H, Yazdi Y, Li X. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging. 1991;13:111-134. [PubMed] [Cited in This Article: ] |
| 108. | Foucher J, Castéra L, Bernard PH, Adhoute X, Laharie D, Bertet J, Couzigou P, de Lédinghen V. Prevalence and factors associated with failure of liver stiffness measurement using FibroScan in a prospective study of 2114 examinations. Eur J Gastroenterol Hepatol. 2006;18:411-412. [PubMed] [Cited in This Article: ] |
| 109. | Friedrich-Rust M, Wunder K, Kriener S, Sotoudeh F, Richter S, Bojunga J, Herrmann E, Poynard T, Dietrich CF, Vermehren J. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology. 2009;252:595-604. [PubMed] [Cited in This Article: ] |
| 110. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. [PubMed] [Cited in This Article: ] |
| 111. | Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Lédinghen V, Marcellin P, Dhumeaux D, Trinchet JC. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48-54. [PubMed] [Cited in This Article: ] |
| 112. | Arena U, Vizzutti F, Abraldes JG, Corti G, Stasi C, Moscarella S, Milani S, Lorefice E, Petrarca A, Romanelli RG. Reliability of transient elastography for the diagnosis of advanced fibrosis in chronic hepatitis C. Gut. 2008;57:1288-1293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 217] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 113. | Sirli R, Sporea I, Bota S, Popescu A, Cornianu M. A comparative study of non-invasive methods for fibrosis assessment in chronic HCV infection. Hepat Mon. 2010;10:88-94. [PubMed] [Cited in This Article: ] |
| 114. | Kim SU, Jang HW, Cheong JY, Kim JK, Lee MH, Kim DJ, Yang JM, Cho SW, Lee KS, Choi EH. The usefulness of liver stiffness measurement using FibroScan in chronic hepatitis C in South Korea: a multicenter, prospective study. J Gastroenterol Hepatol. 2011;26:171-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 115. | Marcellin P, Ziol M, Bedossa P, Douvin C, Poupon R, de Lédinghen V, Beaugrand M. Non-invasive assessment of liver fibrosis by stiffness measurement in patients with chronic hepatitis B. Liver Int. 2009;29:242-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 364] [Cited by in F6Publishing: 400] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 116. | Kim do Y, Kim SU, Ahn SH, Park JY, Lee JM, Park YN, Yoon KT, Paik YH, Lee KS, Chon CY. Usefulness of FibroScan for detection of early compensated liver cirrhosis in chronic hepatitis B. Dig Dis Sci. 2009;54:1758-1763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 117. | Chan HL, Wong GL, Choi PC, Chan AW, Chim AM, Yiu KK, Chan FK, Sung JJ, Wong VW. Alanine aminotransferase-based algorithms of liver stiffness measurement by transient elastography (Fibroscan) for liver fibrosis in chronic hepatitis B. J Viral Hepat. 2009;16:36-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 308] [Cited by in F6Publishing: 314] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 118. | Kim SU, Kim do Y, Park JY, Lee JH, Ahn SH, Kim JK, Paik YH, Lee KS, Chon CY, Choi EH. How can we enhance the performance of liver stiffness measurement using FibroScan in diagnosing liver cirrhosis in patients with chronic hepatitis B? J Clin Gastroenterol. 2010;44:66-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 119. | Yoneda M, Yoneda M, Mawatari H, Fujita K, Endo H, Iida H, Nozaki Y, Yonemitsu K, Higurashi T, Takahashi H. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with nonalcoholic fatty liver disease (NAFLD). Dig Liver Dis. 2008;40:371-378. [PubMed] [Cited in This Article: ] |
| 120. | Nobili V, Vizzutti F, Arena U, Abraldes JG, Marra F, Pietrobattista A, Fruhwirth R, Marcellini M, Pinzani M. Accuracy and reproducibility of transient elastography for the diagnosis of fibrosis in pediatric nonalcoholic steatohepatitis. Hepatology. 2008;48:442-448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 297] [Cited by in F6Publishing: 283] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 121. | Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, Choi PC, Kowo M, Chan AW, Merrouche W. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 876] [Cited by in F6Publishing: 876] [Article Influence: 62.6] [Reference Citation Analysis (1)] |
| 122. | Obara N, Ueno Y, Fukushima K, Nakagome Y, Kakazu E, Kimura O, Wakui Y, Kido O, Ninomiya M, Kogure T. Transient elastography for measurement of liver stiffness measurement can detect early significant hepatic fibrosis in Japanese patients with viral and nonviral liver diseases. J Gastroenterol. 2008;43:720-728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 123. | Chang PE, Lui HF, Chau YP, Lim KH, Yap WM, Tan CK, Chow WC. Prospective evaluation of transient elastography for the diagnosis of hepatic fibrosis in Asians: comparison with liver biopsy and aspartate transaminase platelet ratio index. Aliment Pharmacol Ther. 2008;28:51-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 124. | Sasso M, Beaugrand M, de Ledinghen V, Douvin C, Marcellin P, Poupon R, Sandrin L, Miette V. Controlled attenuation parameter (CAP): a novel VCTE™ guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36:1825-1835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 564] [Cited by in F6Publishing: 555] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 125. | de Lédinghen V, Vergniol J, Foucher J, Merrouche W, le Bail B. Non-invasive diagnosis of liver steatosis using controlled attenuation parameter (CAP) and transient elastography. Liver Int. 2012;32:911-918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 248] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 126. | Myers RP, Pollett A, Kirsch R, Pomier-Layrargues G, Beaton M, Levstik M, Duarte-Rojo A, Wong D, Crotty P, Elkashab M. Controlled Attenuation Parameter (CAP): a noninvasive method for the detection of hepatic steatosis based on transient elastography. Liver Int. 2012;32:902-910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 234] [Cited by in F6Publishing: 240] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 127. | Castera L, Pinzani M, Bosch J. Non invasive evaluation of portal hypertension using transient elastography. J Hepatol. 2012;56:696-703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 220] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 128. | Robic MA, Procopet B, Métivier S, Péron JM, Selves J, Vinel JP, Bureau C. Liver stiffness accurately predicts portal hypertension related complications in patients with chronic liver disease: a prospective study. J Hepatol. 2011;55:1017-1024. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 236] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 129. | Kazemi F, Kettaneh A, N’kontchou G, Pinto E, Ganne-Carrie N, Trinchet JC, Beaugrand M. Liver stiffness measurement selects patients with cirrhosis at risk of bearing large oesophageal varices. J Hepatol. 2006;45:230-235. [PubMed] [Cited in This Article: ] |
| 130. | Lemoine M, Katsahian S, Ziol M, Nahon P, Ganne-Carrie N, Kazemi F, Grando-Lemaire V, Trinchet JC, Beaugrand M. Liver stiffness measurement as a predictive tool of clinically significant portal hypertension in patients with compensated hepatitis C virus or alcohol-related cirrhosis. Aliment Pharmacol Ther. 2008;28:1102-1110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 131. | Kim BK, Han KH, Park JY, Ahn SH, Kim JK, Paik YH, Lee KS, Chon CY, Kim do Y. A liver stiffness measurement-based, noninvasive prediction model for high-risk esophageal varices in B-viral liver cirrhosis. Am J Gastroenterol. 2010;105:1382-1390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 132. | Kim BK, Kim do Y, Han KH, Park JY, Kim JK, Paik YH, Lee KS, Chon CY, Ahn SH. Risk assessment of esophageal variceal bleeding in B-viral liver cirrhosis by a liver stiffness measurement-based model. Am J Gastroenterol. 2011;106:1654-1662, 1730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 133. | Berzigotti A, Seijo S, Arena U, Abraldes JG, Vizzutti F, García-Pagán JC, Pinzani M, Bosch J. Elastography, spleen size, and platelet count identify portal hypertension in patients with compensated cirrhosis. Gastroenterology. 2013;144:102-111.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 349] [Cited by in F6Publishing: 342] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 134. | Crespo G, Fernández-Varo G, Mariño Z, Casals G, Miquel R, Martínez SM, Gilabert R, Forns X, Jiménez W, Navasa M. ARFI, FibroScan, ELF, and their combinations in the assessment of liver fibrosis: a prospective study. J Hepatol. 2012;57:281-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 135. | Bota S, Herkner H, Sporea I, Salzl P, Sirli R, Neghina AM, Peck-Radosavljevic M. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int. 2013;33:1138-1147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 311] [Cited by in F6Publishing: 292] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 136. | Billiau A. The mode of action of interferons in viral infections and their possible role in the control of hepatitis B. J Hepatol. 1986;3 Suppl 2:S171-S179. [PubMed] [Cited in This Article: ] |
| 137. | Vermehren J, Polta A, Zimmermann O, Herrmann E, Poynard T, Hofmann WP, Bojunga J, Sarrazin C, Zeuzem S, Friedrich-Rust M. Comparison of acoustic radiation force impulse imaging with transient elastography for the detection of complications in patients with cirrhosis. Liver Int. 2012;32:852-858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 138. | Bavu E, Gennisson JL, Couade M, Bercoff J, Mallet V, Fink M, Badel A, Vallet-Pichard A, Nalpas B, Tanter M. Noninvasive in vivo liver fibrosis evaluation using supersonic shear imaging: a clinical study on 113 hepatitis C virus patients. Ultrasound Med Biol. 2011;37:1361-1373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 330] [Cited by in F6Publishing: 281] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 139. | Colecchia A, Montrone L, Scaioli E, Bacchi-Reggiani ML, Colli A, Casazza G, Schiumerini R, Turco L, Di Biase AR, Mazzella G. Measurement of spleen stiffness to evaluate portal hypertension and the presence of esophageal varices in patients with HCV-related cirrhosis. Gastroenterology. 2012;143:646-654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 359] [Cited by in F6Publishing: 338] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 140. | Takuma Y, Nouso K, Morimoto Y, Tomokuni J, Sahara A, Toshikuni N, Takabatake H, Shimomura H, Doi A, Sakakibara I. Measurement of spleen stiffness by acoustic radiation force impulse imaging identifies cirrhotic patients with esophageal varices. Gastroenterology. 2013;144:92-101.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 177] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 141. | Sharma P, Mishra SR, Kumar M, Sharma BC, Sarin SK. Liver and spleen stiffness in patients with extrahepatic portal vein obstruction. Radiology. 2012;263:893-899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 142. | Masuzaki R, Tateishi R, Yoshida H, Goto E, Sato T, Ohki T, Imamura J, Goto T, Kanai F, Kato N. Prospective risk assessment for hepatocellular carcinoma development in patients with chronic hepatitis C by transient elastography. Hepatology. 2009;49:1954-1961. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 292] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 143. | Jung KS, Kim SU, Ahn SH, Park YN, Kim do Y, Park JY, Chon CY, Choi EH, Han KH. Risk assessment of hepatitis B virus-related hepatocellular carcinoma development using liver stiffness measurement (FibroScan). Hepatology. 2011;53:885-894. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 288] [Cited by in F6Publishing: 284] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 144. | Reiberger T, Ferlitsch A, Payer BA, Pinter M, Homoncik M, Peck-Radosavljevic M. Non-selective β-blockers improve the correlation of liver stiffness and portal pressure in advanced cirrhosis. J Gastroenterol. 2012;47:561-568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 145. | Nedredal GI, Yin M, McKenzie T, Lillegard J, Luebke-Wheeler J, Talwalkar J, Ehman R, Nyberg SL. Portal hypertension correlates with splenic stiffness as measured with MR elastography. J Magn Reson Imaging. 2011;34:79-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 146. | Yu JS, Shim JH, Chung JJ, Kim JH, Kim KW. Double contrast-enhanced MRI of viral hepatitis-induced cirrhosis: correlation of gross morphological signs with hepatic fibrosis. Br J Radiol. 2010;83:212-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 147. | Aguirre DA, Behling CA, Alpert E, Hassanein TI, Sirlin CB. Liver fibrosis: noninvasive diagnosis with double contrast material-enhanced MR imaging. Radiology. 2006;239:425-437. [PubMed] [Cited in This Article: ] |
| 148. | Awaya H, Mitchell DG, Kamishima T, Holland G, Ito K, Matsumoto T. Cirrhosis: modified caudate-right lobe ratio. Radiology. 2002;224:769-774. [PubMed] [Cited in This Article: ] |
| 149. | Ahn JH, Yu JS, Hwang SH, Chung JJ, Kim JH, Kim KW. Nontumorous arterioportal shunts in the liver: CT and MRI findings considering mechanisms and fate. Eur Radiol. 2010;20:385-394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 150. | Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology. 1992;16:1343-1349. [PubMed] [Cited in This Article: ] |
| 151. | Dell'era A, Bosch J. Review article: the relevance of portal pressure and other risk factors in acute gastro-oesophageal variceal bleeding. Aliment Pharmacol Ther. 2004;20 Suppl 3:8-15; discussion 16-17. [PubMed] [Cited in This Article: ] |
| 152. | Kim H, Choi D, Gwak GY, Lee JH, Park MK, Lee HIe, Kim SH, Nam S, Yoo EY, Do YS. Evaluation of esophageal varices on liver computed tomography: receiver operating characteristic analyses of the performance of radiologists and endoscopists. J Gastroenterol Hepatol. 2009;24:1534-1540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 153. | Yu NC, Margolis D, Hsu M, Raman SS, Lu DS. Detection and grading of esophageal varices on liver CT: comparison of standard and thin-section multiplanar reconstructions in diagnostic accuracy. AJR Am J Roentgenol. 2011;197:643-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 154. | Kim SH, Kim YJ, Lee JM, Choi KD, Chung YJ, Han JK, Lee JY, Lee MW, Han CJ, Choi JI. Esophageal varices in patients with cirrhosis: multidetector CT esophagography--comparison with endoscopy. Radiology. 2007;242:759-768. [PubMed] [Cited in This Article: ] |
| 155. | Oliphant TE, Manduca A, Ehman RL, Greenleaf JF. Complex-valued stiffness reconstruction for magnetic resonance elastography by algebraic inversion of the differential equation. Magn Reson Med. 2001;45:299-310. [PubMed] [Cited in This Article: ] |
| 156. | Yin M, Talwalkar JA, Glaser KJ, Manduca A, Grimm RC, Rossman PJ, Fidler JL, Ehman RL. Assessment of hepatic fibrosis with magnetic resonance elastography. Clin Gastroenterol Hepatol. 2007;5:1207-1213.e2. [PubMed] [Cited in This Article: ] |
| 157. | Huwart L, Sempoux C, Salameh N, Jamart J, Annet L, Sinkus R, Peeters F, ter Beek LC, Horsmans Y, Van Beers BE. Liver fibrosis: noninvasive assessment with MR elastography versus aspartate aminotransferase-to-platelet ratio index. Radiology. 2007;245:458-466. [PubMed] [Cited in This Article: ] |
| 158. | Huwart L, Sempoux C, Vicaut E, Salameh N, Annet L, Danse E, Peeters F, ter Beek LC, Rahier J, Sinkus R. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology. 2008;135:32-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 547] [Cited by in F6Publishing: 510] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 159. | Asbach P, Klatt D, Schlosser B, Biermer M, Muche M, Rieger A, Loddenkemper C, Somasundaram R, Berg T, Hamm B. Viscoelasticity-based staging of hepatic fibrosis with multifrequency MR elastography. Radiology. 2010;257:80-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 174] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 160. | Wang Y, Ganger DR, Levitsky J, Sternick LA, McCarthy RJ, Chen ZE, Fasanati CW, Bolster B, Shah S, Zuehlsdorff S. Assessment of chronic hepatitis and fibrosis: comparison of MR elastography and diffusion-weighted imaging. AJR Am J Roentgenol. 2011;196:553-561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 181] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 161. | Wang QB, Zhu H, Liu HL, Zhang B. Performance of magnetic resonance elastography and diffusion-weighted imaging for the staging of hepatic fibrosis: A meta-analysis. Hepatology. 2012;56:239-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 196] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 162. | Forns X, Ampurdanès S, Llovet JM, Aponte J, Quintó L, Martínez-Bauer E, Bruguera M, Sánchez-Tapias JM, Rodés J. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36:986-992. [PubMed] [Cited in This Article: ] |
| 163. | Adams LA, Bulsara M, Rossi E, DeBoer B, Speers D, George J, Kench J, Farrell G, McCaughan GW, Jeffrey GP. Hepascore: an accurate validated predictor of liver fibrosis in chronic hepatitis C infection. Clin Chem. 2005;51:1867-1873. [PubMed] [Cited in This Article: ] |
| 164. | Calès P, Oberti F, Michalak S, Hubert-Fouchard I, Rousselet MC, Konaté A, Gallois Y, Ternisien C, Chevailler A, Lunel F. A novel panel of blood markers to assess the degree of liver fibrosis. Hepatology. 2005;42:1373-1381. [PubMed] [Cited in This Article: ] |
| 165. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [PubMed] [Cited in This Article: ] |
| 166. | Hong WK, Kim MY, Baik SK, Shin SY, Kim JM, Kang YS, Lim YL, Kim YJ, Cho YZ, Hwang HW. The usefulness of non-invasive liver stiffness measurements in predicting clinically significant portal hypertension in cirrhotic patients: Korean data. Clin Mol Hepatol. 2013;19:370-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 167. | Moon KM, Kim G, Baik SK, Choi E, Kim MY, Kim HA, Cho MY, Shin SY, Kim JM, Park HJ. Ultrasonographic scoring system score versus liver stiffness measurement in prediction of cirrhosis. Clin Mol Hepatol. 2013;19:389-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |